-
Posts
14311 -
Joined
-
Last visited
-
Days Won
192
Content Type
Profiles
Forums
Events
Blogs
Gallery
Everything posted by Sazerac
-
-10 Drink more water, Doreen ! and me too.
-
That was an incredible find, @notsmokinjo, Archie and Paul. What a song ! I searched for a smooth segue and the only song that could come up to snuff was the Lennon one.
-
Welcome, MichelleP, Nice quit you have !
-
Here is our friend, Joel Spitzer's A Safer Way To Smoke ? (text and further resources)
-
The USA's FDA has just approved reduced nicotine cigarettes. Here we go again...nicotine manipulation anyone ??? For Immediate Release: December 17, 2019 The U.S. Food and Drug Administration today announced it has authorized the marketing of two new tobacco products manufactured by 22nd Century Group Inc. – Moonlight and Moonlight Menthol, which are combusted, filtered cigarettes that contain a reduced amount of nicotine compared to typical commercial cigarettes. Following a rigorous science-based review of the premarket tobacco product applications (PMTAs) submitted by the company, the agency determined that authorizing these reduced nicotine products for sale in the U.S. is appropriate for the protection of the public health because of, among several key considerations, the potential to reduce nicotine dependence in addicted adult smokers, who may also benefit from decreasing nicotine exposure and cigarette consumption. The agency determined that non-smokers, including youth, are also unlikely to start using the products, and those who experiment are less likely to become addicted than people who experiment with conventional cigarettes. While today’s action permits the new tobacco products to be legally sold or distributed in the U.S., it does not mean these products are safe or “FDA-approved.” In its decision, the agency notes that the Moonlight and Moonlight Menthol cigarette products differ from conventional cigarettes in nicotine content only – the products share similar adverse health risks as conventional cigarettes. There are no safe tobacco products and those who do not use tobacco products should not start. “Conventional cigarettes are designed to create and sustain addiction to nicotine. In announcing the FDA’s comprehensive plan to regulate tobacco and nicotine in July 2017, we noted our commitment to taking actions that will allow more addicted smokers to reduce their dependence and decrease the likelihood that future generations will become addicted to cigarettes,” said Mitch Zeller, J.D., director of the FDA’s Center for Tobacco Products. “Today’s authorization represents the first product to successfully demonstrate the potential for these types of tobacco products to help reduce nicotine dependence among addicted smokers. Still, we must remain vigilant to ensure that the products authorized today actually deliver on that promise for consumers. We’ll be closely monitoring how Moonlight and Moonlight Menthol are marketed and will take action as necessary if the company fails to comply with any applicable statutory or regulatory requirements or if there is a notable increase in the number of non-smokers, including youth, using these products.” Under the PMTA pathway, manufacturers must demonstrate to the agency, among other things, that marketing of the new tobacco product would be appropriate for the protection of the public health. That standard requires the FDA to consider the risks and benefits to the population as a whole, including users and non-users of tobacco products. Importantly, this includes youth. The agency’s evaluation includes reviewing a tobacco product’s components, ingredients, additives and health risks, as well as how the product is manufactured, packaged and labeled. On average, conventional cigarettes made in the U.S. contain tobacco with a nicotine content of 10 to 14 milligrams (mg) per cigarette. Cigarettes with reduced nicotine content, which at the low end may range from 0.4 to 7.4 mg per cigarette, have existed for decades, primarily for research use. Moonlight and Moonlight Menthol have nicotine content between 0.2 to 0.7 mg per cigarette. As part of the FDA’s scientific review, the agency considered whether smokers who switch to reduced nicotine cigarettes would either smoke more cigarettes or change the way they smoked, such as taking a bigger puff, in order to get the same level of nicotine they would have from smoking conventional cigarettes. Generally, the FDA determined that smokers of reduced nicotine cigarettes tend to actually decrease the number of cigarettes smoked per day and that they do not change the intensity of their puff or inhalation. In addition, though data is limited, the agency expects that non-smokers, including youth, are unlikely to start using the authorized products, and those that do experiment with the authorized products are unlikely to develop nicotine dependence. Since Moonlight and Moonlight Menthol meet the definition of a cigarette under the Federal Food, Drug and Cosmetic Act, the company must adhere to existing restrictions for cigarettes under FDA regulations, as well as other federal laws that, among other things, prohibit television and radio advertising. In addition, to further limit youth access to the products and exposure to their advertising and promotion, the FDA is placing stringent restrictions on how the products are marketed – particularly via websites and through social media platforms – by including restrictions that prevent advertising from being targeted to youth. The agency has previously issued a document providing its rationale for these types of postmarket requirements, which highlight important considerations for reviewing the company’s applications. With the authorization of these products, the FDA will evaluate new available data regarding real-world use of these products through postmarketing records and reports as required in the marketing order. The company is required to report regularly to the FDA with information regarding the products on the market, including, but not limited to, ongoing and completed consumer research studies, advertising, marketing plans, sales data, information on current and new users, manufacturing changes and adverse experiences. The FDA may withdraw this marketing order if, among other reasons, it determines that the continued marketing of a product is no longer appropriate for the protection of the public health, such as if there is an uptake of the product by youth. Additionally, today’s action is not a decision on the separate modified risk tobacco product (MRTP) applications that the company submitted to market these products with specific modified risk information. The FDA is continuing its substantive review of the company’s MRTP applications, which includes, among other things, evaluating whether the modified risk advertising or labeling enables the public to comprehend the modified risk information and to understand its relative significance in the context of total health. The company would need to receive an MRTP order from the FDA before they could market a tobacco product with any implicit or explicit claims that, among other things, a product reduces exposure to a substance or that use of the product is less harmful or would reduce harm or the risk of disease associated with other commercially marketed tobacco products. If a company markets a tobacco product as an MRTP without authorization, the company would be in violation of the law and may face FDA advisory or enforcement actions. The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
- 1 reply
-
- 1
-

-
NOPE NOPE NOPE NOPE NOPE
-
-7 Hello festive nicotine free creatures
-
NOPE NOPE NOPE NOPE NOPE
-
Doreen went The Wrong Way and Nancy followed. Dnot -6 (punishment will resume even w/ your 'misunderstanding', darling) Doreen -7 (welcome home !) Nancy -8 (happy holidays, Nancy) Saz -9 (hello darling smoke free creatures)
-
Leaning towards health
-

Out of nowhere BAM - Serious cravings
Sazerac replied to HeatherDianne's topic in Quit Smoking Discussions
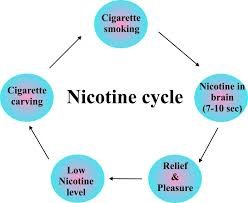
I need to address this, OMC. The 'pleasure' you speak of was not real. It was only feeding the addiction. The satisfaction was not real. It was relieving the crave by feeding the addiction. These are the basic tenets of understanding nicotine addiction ! How Nicotine Works The 'Pleasure' you speak of was a big fat LIE. Nicotine gangstered the pleasure receptors in our brain. Claiming them back provides real pleasure and phenomenal personal power. Nothing sucks about quitting smoking. Nothing. -
-

Out of nowhere BAM - Serious cravings
Sazerac replied to HeatherDianne's topic in Quit Smoking Discussions
Quitting smoking may trigger all sorts of unfamiliar as well as familiar feelings. Add this to an emotional time of year and well....you can expect feeling different than usual. You are a new you ! Once the quit settles down you will grow into your newness with grace and joy. Do not worry. Your 'happy' will return. Be kind with yourself and lavish rewards. S -
-6 Have you sticks thought about your punishment for cheating yet ?
-

Out of nowhere BAM - Serious cravings
Sazerac replied to HeatherDianne's topic in Quit Smoking Discussions
You have a young and precious quit going. Protect it with your life! If you harbor jealousy when seeing people smoke or resentment because you choose not to smoke, PLEASE take some more time and learn more about nicotine addiction. Give yourself rewards for every thought/crave/trigger conquered. Check out this thread, Red Flags. You don't smoke ! You need to be completely clear and stalwart in your choice to put your addiction to Sleep. As time goes by smokey thoughts fade into oblivion but, they do this so subtlety that it is hard to track. Do not loose hope or your commitment, you are winning. -
Welcome, Sweets and congratulations on your first two days of Freedom. You may find this thread helpful 10 Ways To Effectively Use This Forum To Stop Using Nicotine Remember, even if you experience craves and triggers these are not commands to smoke ! Just your brain re-calibrating. You quit smoking !
-
So.....now you are wooing me ? That's adorable Just remember who you may be dealing with, Mighty Mighty Mighty One.
-
Locating your residence is critical. Yours
-
abhorrent neocon tantrums
-
^^^^^^^ I see the cheating sticks are into self flagellation today, tee hee hee. and now back to our regularly festive programming
-
How can you say that and submit me to the full on horror show of Cliff Richard. GAH ! Your town sounds awfully (ahem) nice, Mighty One, but, I think I will stay put and bask in the hoodoo of Art Neville and the Brothers. Please know I have a small 'collection' of alligator, cat, mice, rat, goat, dog, pig and human teeth and assortment of potions etc. I may have the opportunity to put them to use. Be good, bebe. Be good. When in doubt, be exceptionally good. Love, Saz